AMP-ASCO-CAP Recommendations: Simplified Variant Classification
The AMP-ASCO-CAP guidelines provide a structured four-tier system to classify cancer-related genetic (somatic) variants based on clinical and genomic evidence. These classifications help prioritize genetic findings for clinical actionability. Here's a breakdown of the evidence levels:
1. Group A:
- Highest level of evidence.
- Includes genetic variants referenced in professional oncology guidelines or those linked to FDA-approved cancer therapies for specific tumor types.
2. Group B:
- Strong evidence from well-powered studies with expert consensus on gene-tumor associations.
3. Group C:
- Emerging evidence.
- Includes drugs in clinical trials for specific cancers, FDA-approved drugs for different tumor types, or findings from small studies with limited consensus.
4. Group D:
- Preliminary evidence.
- Data from preclinical trials or early publications without consensus on gene-tumor relevance.
Genomic Oncology Learning Database (GOLD)
We implemented the AMP-ASCO-CAP guidelines into a computational framework that simplifies the interpretation of somatic cancer variants. Using this adaptive algorithm, we annotated somatic variants from publicly available datasets to create clinically actionable somatic variants set for cancer genomic. This dataset focuses on variants that are compatible with most NGS platforms and prioritized for clinical utility.
Key Features:
- Streamlined variant classification based on AMP-ASCO-CAP evidence tiers.
- A curated list of variants with actionable insights for cancer treatment and research.
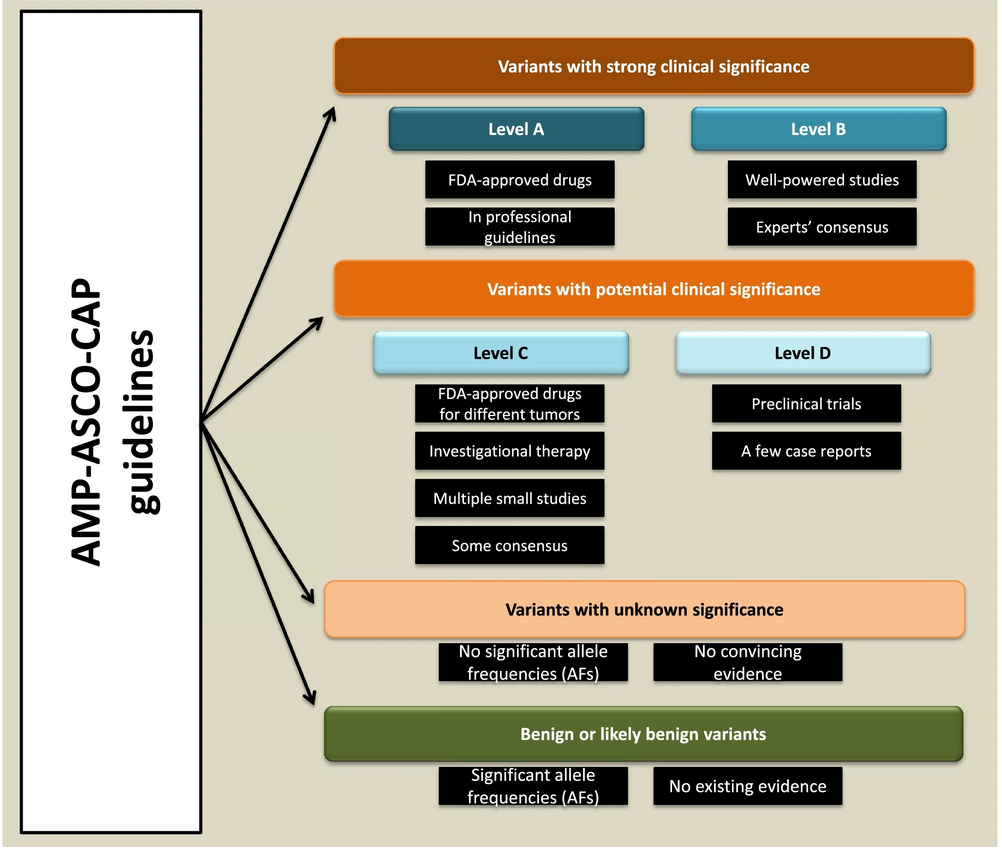
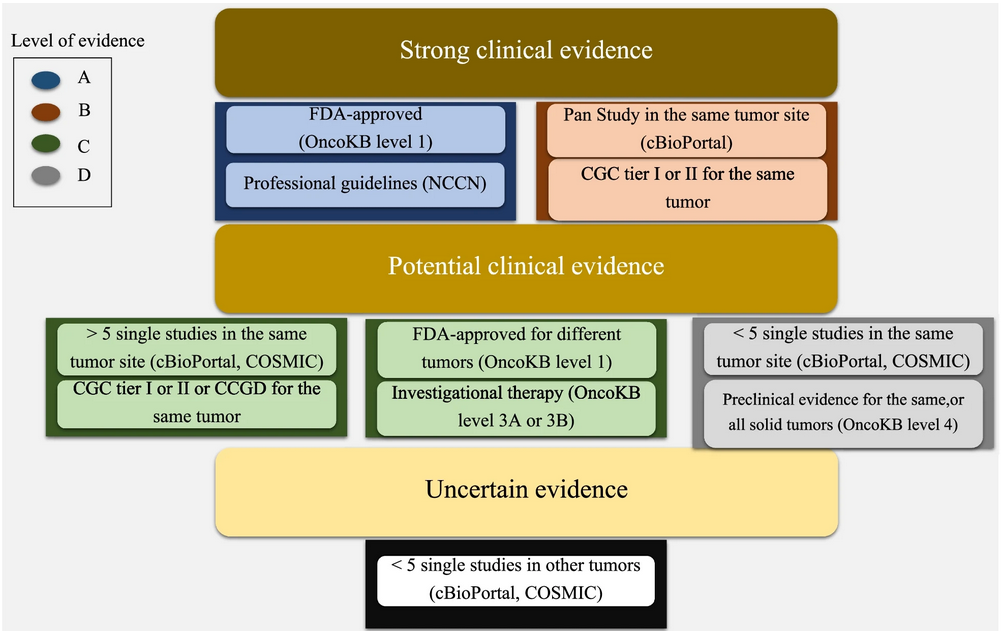
Figures provide visual representations of the classification system and computational workflow, helping users navigate and utilize the tool effectively.
